Workings of a 3He refrigerator
Please note that most of the graphics here are clickable.
Let us start with a simpler example first. How would you cool something to
very low temperature. Well, the easiest thing is
to find some substance that is already at that low temperature and bring your
sample in thermal contact with it. You all know that
one of such substances is liquid nitrogen.
Liquid nitrogen has boiling point of about 77 K. Quite
a bit more expensive, but much more useful liquid in low
temperature physics is helium with its boiling temperature
of just 4.2 K. So, what can be easier: let's build a container into which
we can put our sample and then pour liquid helium on
top. If only things were this simple! Just like water in a tea pot,
helium will boil when put into contact with anything that is warmer
than those 4.2 degrees. Not only direct contact, but even heat transfer
through radiation will blow all your helium (and a lot of
your funding) off if, of course, you don't take certain precautions.
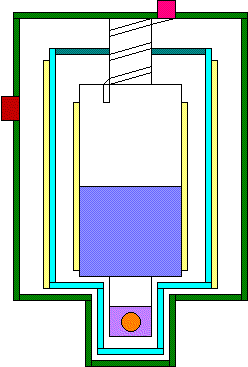 Look at the diagram to the right. This is a simple single-stage vapor-cooled
4He cryostat.
You can see three distinct walls. The outer wall is a thick stainless steel
container, with pump and
pressure gauge ports. The medium wall is an aluminum shield wrapped
with about 50 layers of aluminized mylar.
The innermost wall is
the container of 4He, or 4He can. It is also covered with several
layers of aluminized mylar.
Look at the diagram to the right. This is a simple single-stage vapor-cooled
4He cryostat.
You can see three distinct walls. The outer wall is a thick stainless steel
container, with pump and
pressure gauge ports. The medium wall is an aluminum shield wrapped
with about 50 layers of aluminized mylar.
The innermost wall is
the container of 4He, or 4He can. It is also covered with several
layers of aluminized mylar.
The volume between the outer and the inner walls (so called OVC --
outer vacuum chamber)
has to be pumped out to low pressure (~10^(-7) torr) to reduce
conductive heat exchange. The so called "Superinsulating" layers of
mylar serve a double function: they help to cut off the thermal radiation
flux
and to intercept hot molecules that would otherwise hit the 4He container.
When 4He is put inside, it inevitably boils off. Cold gas, before escaping
into the atmosphere
(we do not recycle He yet), has to pass through the bellows thermally
anchored to the top of the aluminum shield
and cools it to about 100 K.
This gives the name vapor-cooled to this cryostat design. Usually,
if the space and weight
allow, another volume connected to the aluminum shield is introduced.
That volume can be
seperately filled up with liquid nitrogen. Those systems require somewhat
smaller amount of
helium to operate.
We are all set. Now we can put the sample somewhere in the helium can, or
connect it to the
can with a thermal link, drill some holes (windows) for the x-rays
(and patch them with something
that lets the x-rays through but still keeps the vacuum -- Kapton and
beryllium are prime
materials for that), and that's it. As long as we pour in (or transfer)
more helium every
24 hours or so, the cryostat is going to work. There are of course minor
details like thermometry
and such, which require several wires to run down the cryostat to the
position of the sample.
But what if we want to cool even more? The difference between 4.2 K and
1 K for a
physicist is not just 3.2 degrees, but a ratio of 4.2. In this sense
it is somewhat like the difference
between room temperature (300 K) and the melting point of copper (1356 K).
You may know that the boiling temperature of a liquid is a function of
its vapor pressure.
It means that just by pumping on a liquid it is possible to cool it
(and the sample) to
the extent to which you can reduce the pressure. This allows to cool
helium to
about 1 K without any further complications. The only problem is that
while you are cooling
the liquid itself, you have to evaporate a sizable portion of it.
By the time you get
to 1.2 K, almost half of your helium is gone! Moreover, if you want to
add some helium,
you have to warm up to 4 K and then cool down again wasting a lot of
helium on this unnecessary cycling.
So, we come to our next more complicated cryostat. The outside
looks just the same,
but we create another vacuum volume in the very center of
our previous design, and
introduce the 1 Kelvin Pot.
It appears that continuously cooling just a small part of all helium to really
low temperature does not require as much helium. The pump is connected
to the 1K pot,
which is being fed by helium through a thin long capillary (impedance).
The experimental
cell (or sample) is then thermally connected to the 1K pot.
Note, that this configuration
allows non-interrupting helium fill-ups.
Although it is called the 1K pot, I have never seen one running
lower than 1.1K.
The limit of 1 Kelvin is due to the fact that 4He becomes superfluid below 2.17 K.
A thin
film of superfluid helium creeps up the pump line from a cold spot to a warm
spot where it evaporates.
Because of this, the pressure in the pump line is determined by this
warmer place, higher
in the cryostat, preventing the liquid from cooling further.
With a battery of
fast pumps the lowest temperature ever achieved
this way was ~0.75 K.
Helium has a lighter isotope, 3He. Unlike 4He, it is a fermion and
becomes superfluid
via Cooper pairing only at 1 mK. A cryostat with the 1K pot
running on pure 3He would
reach temperatures of ~0.25 K. Unfortunately, 1 liter of liquid 3He
sells for about
$100000 (it is $1e5). The natural abundance of 3He is extremely low.
The cheapest way to produce it
is to capture gases generated in a nuclear reactor.
It is possible to have 3He in a closed circulation loop. Unfortunately,
a large portion
of 3He will be in the pump lines, making the operation expensive.
A way around it is to
have a completely contained 3He cooling part using a sorption pump.
When cooled, gases generally adsorb to solid
surfaces forming a monolayer or two.
Sorption pump is based on the idea that at ~10 K almost
all of the 3He gets adsorbed,
whereas at ~35 K practically all of the molecules desorb.
Sorption pump is a cylinder that contains materials like
activated charcoal, which have enormous total surface area
(tens of square meters
per gram).
3He is stored in an outside tank.
After the cryostat has been cooled to 1.1 K, the
sorption pump is heated up and 3He is
introduced into the system.
When passing through a cold copper tube in the
1K pot ( the condenser),
it becomes liquid and drips down into the 3He pot.
After the condensation is complete,
the outside tank is closed. The sorption pump
is allowed to cool down through a weak
thermal link to the helium can. 3He gets
adsorbed in the sorption pump reducing the
vapor pressure and lowering the temperature of the 3He pot.
After all of the 3He evaporates,
the sorption pump has to be heated up to condense 3He and so on.
One operation cycle
in Syncryo is about 6 hours long.
Click here to get back to the
X-Ray Group Home Page
 penanen@xray.harvard.edu
penanen@xray.harvard.edu
 Look at the diagram to the right. This is a simple single-stage vapor-cooled
4He cryostat.
You can see three distinct walls. The outer wall is a thick stainless steel
container, with pump and
pressure gauge ports. The medium wall is an aluminum shield wrapped
with about 50 layers of aluminized mylar.
The innermost wall is
the container of 4He, or 4He can. It is also covered with several
layers of aluminized mylar.
Look at the diagram to the right. This is a simple single-stage vapor-cooled
4He cryostat.
You can see three distinct walls. The outer wall is a thick stainless steel
container, with pump and
pressure gauge ports. The medium wall is an aluminum shield wrapped
with about 50 layers of aluminized mylar.
The innermost wall is
the container of 4He, or 4He can. It is also covered with several
layers of aluminized mylar.
 penanen@xray.harvard.edu
penanen@xray.harvard.edu